UDI stands for a unique serial number given to each medical device. In this scenario, each identical device would be assigned its own serial number which becomes easy to track it through out its life cycle.
To understand UDI you must also understand what it is not! . A good scope statement has both inclusion and exclusion criteria. There is a lot of confusion in the literature about UDI
We define three concepts and show how they relate to UDI
- Identification tells us what the device is. The key is the Device Identifier (DI)
- The DI is on the device label and package
- It allows entry into the GUDID which provides important data elements to identify the device
- 820.60 requires, “Each manufacturer shall establish and maintain procedures for identifying product during all stages of receipt, production, distribution, and installation to prevent mix-ups.”
- This aspect of identification focuses on identification at the manufacturer’s location
- UDI focuses on identification after distribution
- With the DI, your customer has access to your device information in the FDA-CDRH database.
- Tracing provides information about the production history of the device
- 820.65 Traceability : Each manufacturer of [certain device types] shall establish and maintain procedures for identifying with a control number each unit, lot, or batch of finished devices and where appropriate components.
- ISO 13485:2016 Clause 7.5.9 Traceability – General :
- The organization shall establish documented procedures for traceability. Such procedures shall define the extent of product traceability and the records required.
- Where traceability is a requirement, the organization shall control and record the unique identification of the product.
- FDA QSR Preamble #120 : It is important that the traceability requirements in Part 820 are not confused with the Medical Device Tracking regulation in Part 821. The tracking regulation is intended to ensure that tracked devices can be traced from the device manufacturing facility to the person for whom the device is indicated, that is, the patient. … In contrast, the traceability provision requires that a device that meets the definition of a “critical device” can be traced from the manufacturing facility only to the “initial consignee” as discussed in Sec. 820.160 Distribution.
- Tracking provides information about the patient who has the device
- Tracking provides information about the patient or user who has the device (See 21 CFR Part 821 for the tracking regulations)
- One goal of UDI is to help populate electronic health records (EHR), but the information is in the EHR.
- The DI+GUDID does not provide information about any patient or facility who may have used the device
UDI Tracking
![]()
Difference Between Tracing & Tracking
![]()
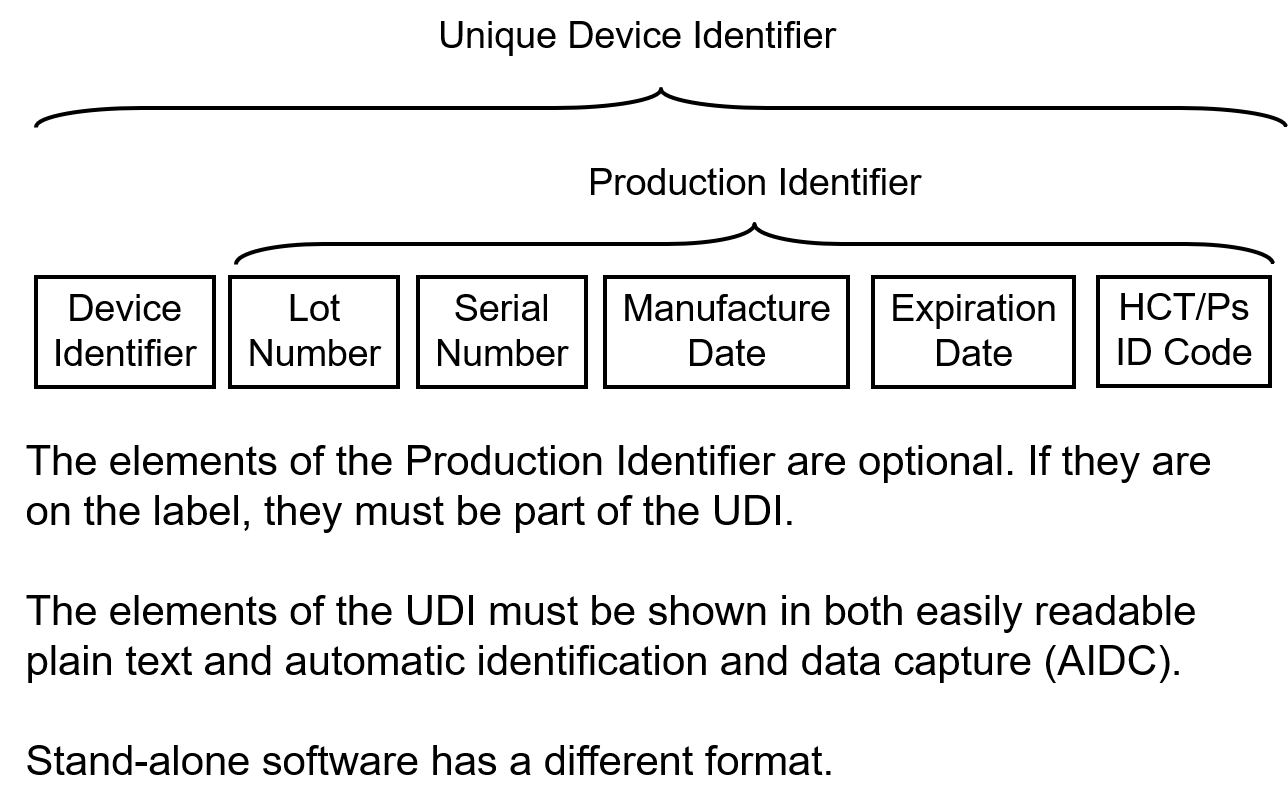
Overview of UDI Elements
UDI Locations
Direct Marking :
- If the device requires direct marking and doesn’t qualify for an exemption:
- Determine if the direct marking DI should be the same or different from the Primary DI
- Determine the method for direct marking so it is permanent
- Obtain a DI from an accredited agency
Label UDI:
- Determine the machine readable format (AIDC) for the UDI
- Obtain a DI from an accredited agency
- Determine the production identifiers (PI) to include on the label
- Change the date format on the label to YYYY-MM-DD
- Not applicable to electronic products regulated by FDA-CDRH
Package UDI:
- Determine the machine readable format (AIDC) for the UDI
- Obtain a DI from an accredited agency
- Change the date format on the package to YYYY-MM-DD
Important Points :
- The UDI system is a powerful tool for device Identification
- The UDI system does not provide information for device Tracking
- The UDI system does not provide information for device Tracing
